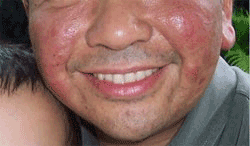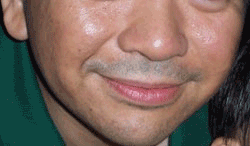
The U.S. Food and Drug Administration today issued a public health advisory concerning three confirmed, and one possible report of progressive multifocal leukoencephalopathy (PML), a rare brain infection, in patients using the psoriasis drug Raptiva (efalizumab). Three of those patients have died. All four patients were treated with the drug for more than three years. None of the patients were receiving other treatments that suppress the immune system.
[Read more…]
US FDA: Psoriasis Drug RAPTIVA could result in serious brain infection and death
February 22, 2009 By 1 Comment